Solar-light-driven CO2 reduction by methane on Pt nanocrystals partially embedded in mesoporous CeO2 nanorods with high light-to-fuel efficiency†
Abstract
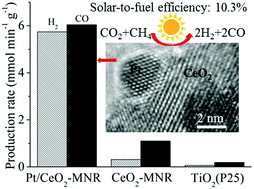
A unique nanocomposite of Pt nanocrystals partially embedded in mesoporous CeO2 nanorods was prepared by a facile method. The nanocomposite exhibits highly efficient catalytic activity and very good durability for CO2 reduction by methane (CRM) under focused solar light. It produces very high fuel production rates of H2 and CO (5.7, 6.0 mmol min−1 g−1) with a very high light-to-fuel efficiency (η, 10.3%). Remarkably, even under the visible-infrared irradiation with wavelengths above 690 nm, it still exhibits efficient catalytic activity with a very high η (10.6%). Based on experimental evidence we demonstrate that the highly efficient catalytic activity arises from a novel efficient solar-light-driven thermocatalytic process of CRM on the nanocomposite. We find a synergetic effect between Pt nanoparticles as catalytically active sites and CeO2 in Pt/CeO2-MNR that significantly improves the catalytic activity and durability. We provide an insight into the synergetic effect based on the evidence of in situ FTIR and isotope labeling: the partial confinement of Pt nanocrystals in mesoporous CeO2 in Pt/CeO2-MNR makes the oxygen of CeO2 at the Pt/CeO2 interface more active due to the metal–support interaction. The active oxygen of CeO2 at the Pt/CeO2 interface not only directly participates in the dissociation of CH4 and the oxidation of the formed CHx species on CeO2, but also migrates through the reverse oxygen spillover to the surface of Pt nanoparticles and participates in the dissociation of CH4 and the oxidation of the formed CHx species on Pt nanoparticles. On the other hand, both the oxygen vacancies in ceria at the Pt/CeO2 interface formed by the oxidation of the CHx species and the Pt sites on the surface of Pt nanoparticles participate in the dissociation of CO2. Meanwhile, the chemisorbed oxygen on the surface of Pt nanoparticles formed by the dissociation of CO2 migrates through the oxygen spillover to ceria, where it replenishes the formed oxygen vacancies and participates in the oxidation of the CHx species on CeO2. The synergetic effect significantly improves the catalytic activity of Pt/CeO2 and inhibits the carbon deposition, thus considerably improving the durability.


 Please wait while we load your content...
Please wait while we load your content...