Metal-free benzannulation of yne-allenone esters for atom economical synthesis of functionalized 1-naphthols†
Abstract
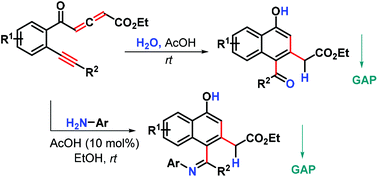
A new metal-free benzannulation reaction of yne-allenone esters with H2O by using HOAc as a Lewis acid promoter is reported, providing an atom economical protocol toward aroyl 1-naphthols with good to excellent yields. Exchanging H2O for aromatic amines resulted in iminated Z-1-naphthols with high stereoselectivity in a highly functional-group-compatible manner under mild conditions. The purification of these products only needs to be carried out by washing with 95% EtOH solvent, thereby avoiding traditional chromatography and recrystallization, which belongs to group-assisted purification (GAP) chemistry.


 Please wait while we load your content...
Please wait while we load your content...