Transformation of lignin model compounds to N-substituted aromatics via Beckmann rearrangement†
Abstract
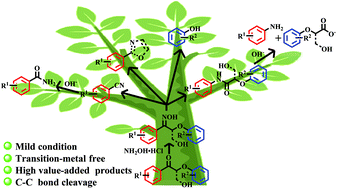
Here we present the highly effective cleavage of C–C bonds in lignin model compounds for the production of N-substituted aromatics in up to 96% total yield, including benzonitriles and amides, via oxime formation followed by Beckmann rearrangement (BR). The amides could be further hydrolyzed to anilines (>92% yield) and carboxylic acids (>90% yield), respectively. In addition, the employment of a substrate with a γ-OH group will lead to the formation of C-2 monosubstituted oxazole. A one-pot process involving the BR reaction and hydrolysis has also been developed to directly afford an up to 96% total yield of benzonitriles, benzamides, and anilines. This strategy enabled us to successfully apply the BR reaction to the degradation of lignin model compounds to N-functionalized aromatic products under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...