Multiple hydrogen bond coordination in three-constituent deep eutectic solvents enhances lignin fractionation from biomass†
Abstract
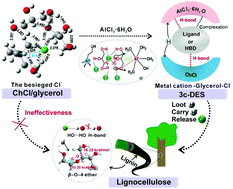
As an emerging generation of green solvents, deep eutectic solvents (DESs) are promising for the pretreatment of lignocellulose and the production of biochemicals. However, not all DESs are effective for the cleavage of lignin–carbohydrate complexes (LCCs) in lignocellulose and the fractionation of lignin. In this study, we analyzed the nature of complex molecular interactions between choline chloride (ChCl) and glycerol in ChCl/glycerol (1 : 2) DES using density functional theory and the Kamlet–Taft solvatochromic method. The ChCl–glycerol DES exhibited weak competing interactions towards the linkages in the LCC network because the intramolecular hydrogen bonds (H-bonds) in ChCl–glycerol were constrained by mutually anionic H-bonds ([Cl(glycerol)]−) and cationic H-bonds ([Ch(glycerol)]+). Furthermore, because of the absence of active protons and acidic sites, the DES was unable to cleave ether bond linkages in the LCCs. Accordingly, we designed a three-constituent DES (3c-DES) by coordinating AlCl3·6H2O in ChCl/glycerol DES based on an acidic multisite coordination theory. The competition of anionic H-bonds and unidentate aluminum ligands was synchronized to form supramolecular complexes, allowing the multisite bridging ligands to cleave both the H-bonds and ether bonds in LCCs. Consequently, the lignin fractionation efficiency was significantly improved from 3.61% to 95.46%, and the lignin purity reached 94 ± 0.45%.


 Please wait while we load your content...
Please wait while we load your content...