Programming cascade reactions interfacing biocatalysis with transition-metal catalysis in Deep Eutectic Solvents as biorenewable reaction media†
Abstract
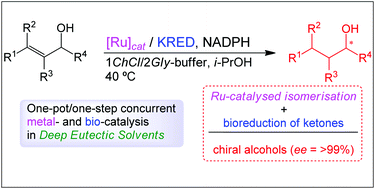
The first application of Deep Eutectic Solvents (DESs) in the asymmetric bioreduction of ketones has been accomplished for purified ketoreductases (KREDs). The performance of the biocatalysts was enhanced by increasing the percentage of neoteric solvent in DES-buffer mixtures. At a buffer content of 50% (w/w) and even 20% (w/w), the combination of either choline chloride (ChCl)/glycerol (Gly) (1 : 2) or ChCl/sorbitol (1 : 1) proved to be most effective for achieving up to >99% conversion and up to >99% enantiomeric excess of the corresponding secondary alcohols. Moreover, this reaction medium was used to perform the first example of a chemoenzymatic cascade process in DES-buffer mixtures, namely the ruthenium-catalysed isomerisation of racemic allylic alcohols coupled with a further enantioselective bioreduction, in both sequential and concurrent modes.
- This article is part of the themed collection: 2018 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...