Controlled mono-olefination versus diolefination of arenes via C–H activation in water: a key role of catalysts†
Abstract
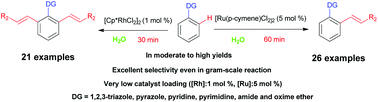
Herein, we report the olefination of arenes via C–H activation in water instead of in expensive and pollution-causing organic solvents. In this process, the catalysts were shown to play a key role in controlling mono- and diolefinations. More specifically, [Ru(p-cymene)Cl2]2 predominantly gave mono-olefinated products while [Cp*RhCl2]2 selectively afforded diolefinated products. Importantly, only relatively small amounts of the catalysts were required ([Ru(p-cymene)Cl2]2 5 mol%, [Cp*RhCl2]2 1 mol%) and the reaction time was significantly shortened from tens of hours using previous strategies to tens of minutes. Additionally, the new methodology was shown to work with a variety of functional groups and olefins, to achieve moderate to good yields of products, and to be applicable to a gram-scale synthesis at a low catalyst loading.


 Please wait while we load your content...
Please wait while we load your content...