CO2 conversion to synthesis gas via DRM on the durable Al2O3/Ni/Al2O3 sandwich catalyst with high activity and stability†
Abstract
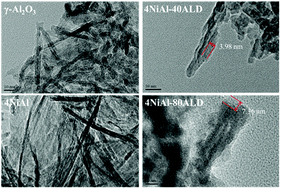
CO2 conversion to synthesis gas with a CO/H2 molar ratio around 1 was realized by using the dry reforming of methane reaction (DRM) at 800 °C. The key problem was to design catalysts with both high activity and strong durability at such a high reaction temperature. This work developed a novel Al2O3/Ni/Al2O3 sandwiched catalyst prepared by coating Al2O3-supported Ni nanoparticles with a porous Al2O3 thin film by atomic layer deposition (ALD). The catalyst with 80 layers of Al2O3 thin films exhibited the highest activity. Both CO2 and CH4 conversions reached nearly 100% with absolute selectivities towards CO and H2. More importantly, this catalyst displayed excellent stability and could be used for more than 400 h in the DRM reaction at 800 °C without significant deactivation. Mechanism analysis revealed that the deactivation mainly resulted from the gathering of Ni nanoparticles at high temperature, corresponding to the decrease of Ni active sites. Moreover, a large-sized Ni active site could easily cause carbon deposition, which could further accelerate the catalyst deactivation. The Al2O3/Ni/Al2O3 sandwiched catalyst could effectively protect Ni nanoparticles from gathering owing to the double strong interactions between the Ni active sites and Al2O3 support.


 Please wait while we load your content...
Please wait while we load your content...