Iron(iii)-catalyzed selective N–O bond cleavage to prepare tetrasubstituted pyridines and 3,5-disubstituted isoxazolines from N-vinyl-α,β-unsaturated ketonitrones†
Abstract
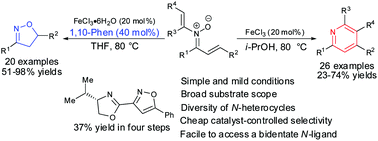
An iron(III)-catalyst controlled cyclization and selective N–O bond cleavage of N-vinyl-α,β-unsaturated nitrones has been achieved under mild conditions to access tetrasubstituted pyridines and 3,5-disubstituted isoxazolines in moderate to good yields. The tetrasubstituted pyridines were afforded with FeCl3 as a catalyst while using FeCl3·6H2O combined with 1,10-phenanthroline delivered isoxazolines. The regioselectivity for cyclization of styrenyl groups in N-vinyl-α,β-unsaturated nitrones was completely different during the formation of pyridines and isoxazolines. A rational mechanism for the formation of pyridines and isoxazolines was proposed based on the further control experimental studies. The isoxazolines can be converted to a novel bidentate N-ligand over four steps and an epoxypyridine scaffold was obtained from N-vinyl nitrone when copper(II) acetate in combination with the prepared bidentate N-ligand was used.


 Please wait while we load your content...
Please wait while we load your content...