Hydration and alkoxylation of alkynes catalyzed by NHC–Au–OTf†
Abstract
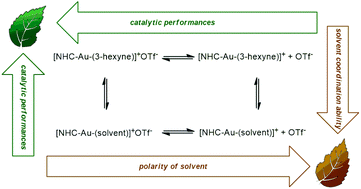
The NHC–Au–OTf [NHC = 1,3-bis(2,6-di-isopropylphenyl)-imidazol-2-ylidene] catalyst was tested in the alkoxylation and hydration of alkynes in a wide set of neoteric solvents for the first time. We found that most of these solvents (ethyl lactate, glycerol, propylene carbonate, D-limonene, γ-valerolactone, and p-cymene) are comparable or better solvents with respect to traditional VOSs. An important beneficial effect of γ-valerolactone was found in the hydration of inactive diphenylacetylene and one order of magnitude higher TOF was obtained with respect to the literature values. Kinetic experiments and DFT calculations seem to indicate that the polarity of the solvent and the presence of suitable proton acceptor/donor functionalities on the solvent itself could be taken into account.


 Please wait while we load your content...
Please wait while we load your content...