Hydrogen generation from formic acid decomposition on a highly efficient iridium catalyst bearing a diaminoglyoxime ligand†
Abstract
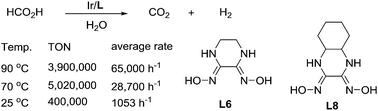
A new iridium catalyst bearing a dioxime derived ligand has been developed for aqueous formic acid (FA) dehydrogenation. This catalyst features high stability and high efficiency for dehydrogenation of FA in aqueous solution without any additives. With the in situ formed catalysts, TONs of up to 3 900 000 (average rate: 65 000 h−1) at 90 °C were achieved. At 70 °C, an even higher TON of 5 020 000 was obtained, which is the highest TON ever reported. More interestingly, this catalyst can also give a TON of 400 000 and an average rate of 1053 h−1 even at room temperature. Electron-rich amine substituents and the dioxime structure of the ligand are beneficial to the high stability and high efficiency of the catalyst.


 Please wait while we load your content...
Please wait while we load your content...