Highly efficient electrochemical reduction of CO2 into formic acid over lead dioxide in an ionic liquid–catholyte mixture†
Abstract
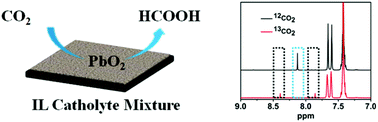
The development of efficient electrocatalytic systems for CO2 conversion with high current density and high Faradaic efficiency simultaneously is crucially important but challenging. Herein, we described the first study of the electrochemical reduction of CO2 into HCOOH over commercial lead dioxide (PbO2) as the electrode in ionic liquid (IL)-containing catholytes. It was found that 1-benzyl-3-methylimidazolium tetrafluoroborate ([Bzmim]BF4) was the best IL to enhance the efficiency of the PbO2 electrode in the electroreduction of CO2 to HCOOH. In a ternary electrolyte consisting of [Bzmim]BF4 (14.7 wt%), H2O (11.7 wt%) and acetonitrile, the PbO2 electrode showed an excellent performance in electroreduction of CO2 into HCOOH with high Faradaic efficiency (95.5%) and very high current density (40.8 mA cm−2) simultaneously.


 Please wait while we load your content...
Please wait while we load your content...