A nitrogen-ligated nickel-catalyst enables selective intermolecular cyclisation of β- and γ-amino alcohols with ketones: access to five and six-membered N-heterocycles†
Abstract
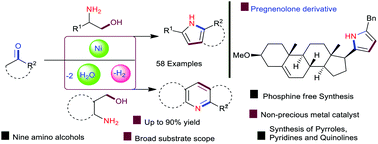
Owing to the great demand for the synthesis of N-heterocycles, development of new reactions that utilise renewable resources and convert them into key chemicals using non-precious base metal-catalysts is highly desirable. Herein, we demonstrated a sustainable Ni-catalysed dehydrogenative approach for the synthesis of pyrroles, pyridines, and quinolines by the reaction of β- and γ-amino alcohols with ketones via C–N and C–C bond formations in a tandem fashion. A variety of aryl, hetero-aryl, and alkyl ketones having free amine, halide, alkyl, alkoxy, alkene, activated benzyl, and pyridine moieties were converted into synthetically interesting 2,3 and 2,3,5-substituted bicyclic as well as tricyclic N-heterocycles with up to 90% yields. As a highlight, we demonstrated the synthesis of an interesting pyrrole derivative by intermolecular cyclisation of a steroid hormone with phenylalaninol.


 Please wait while we load your content...
Please wait while we load your content...