Efficient and reversible absorption of ammonia by cobalt ionic liquids through Lewis acid–base and cooperative hydrogen bond interactions†
Abstract
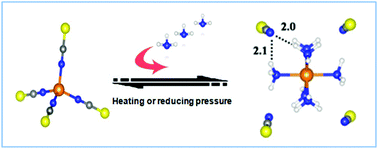
Ammonia (NH3) emissions have caused a wide range of environmental problems and serious harm to human health. However, efficiently separating NH3 and simultaneously recovering high purity NH3 easily remains a great challenge. A new strategy to design transition metal ionic liquids (MILs) by combining specific metal centers and ligands with ILs was proposed for efficient and reversible absorption of NH3. Not only exceptional NH3 absorption capacity and high NH3/CO2 selectivity, but also excellent recyclability were achieved by cobalt ILs [Cnmim]2[Co(NCS)4]. The maximal capacity of NH3 is up to 6.09 mol NH3 mol IL−1 at 30 °C and 0.10 MPa, which is much higher than all reported ILs to date, and is over 30 times higher than the conventional ILs [Cnmim][SCN]. The superior NH3 capacity and desorption performance originate from the moderate Lewis acid–base and cooperative hydrogen bond interactions between the metal center-ligands and NH3.


 Please wait while we load your content...
Please wait while we load your content...