Iodide reagent controlled reaction pathway of iodoperoxidation of alkenes: a high regioselectivity synthesis of α- and β-iodoperoxidates under solvent-free conditions
Abstract
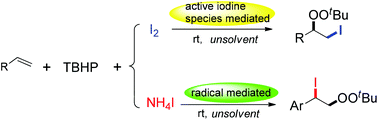
A highly atom economical, efficient and environmentally friendly iodoperoxidation of alkenes with tert-butyl hydroperoxide (TBHP) and iodide/iodine is reported in this paper. This method afforded a convenient path to obtain two different configurations of iodoperoxidates from the same starting materials. Notably, the regiodivergent iodoperoxidation reaction was achieved by using different iodide reagents. A series of control experiments were performed, which suggested the involvement of a radical pathway for the anti-Markovnikov type iodoperoxidates (α) in the combination of ammonium iodide (NH4I)/TBHP, and an active cationic iodine pathway for the Markovnikov type adduct (β) with iodine (I2) and TBHP.


 Please wait while we load your content...
Please wait while we load your content...