Transition metal free oxygenation of 8-aminoquinoline amides in water†
Abstract
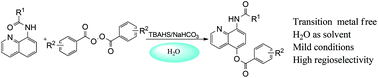
The oxygenation of 8-aminoquinoline amides by benzoyl peroxide at the C5 position in water is developed in the absence of a transition metal catalyst, affording the desired products in moderate to good yields of up to 88%. Mechanism studies reveal that the reaction would involve a radical process.


 Please wait while we load your content...
Please wait while we load your content...