Flavin–iodine coupled organocatalysis for the aerobic oxidative direct sulfenylation of indoles with thiols under mild conditions†
Abstract
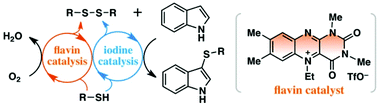
A unique coupled redox organocatalysis system using flavin and iodine catalysts efficiently promoted the metal-free aerobic oxidative direct sulfenylation of indoles with thiols at ambient temperature without any sacrificial reagents, except environmentally benign molecular oxygen. Biomimetic flavin catalysis plays multiple roles in aerobic oxidative transformations, not only regenerating I2 from in situ generated I−, but also converting thiols into disulfides.


 Please wait while we load your content...
Please wait while we load your content...