Rethinking Co(CO3)0.5(OH)·0.11H2O: a new property for highly selective electrochemical reduction of carbon dioxide to methanol in aqueous solution†
Abstract
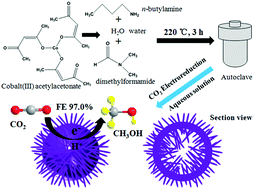
Co(CO3)0.5(OH)·0.11H2O is usually acknowledged and used as a precursor to synthesize other nanomaterials. However, some important properties of Co(CO3)0.5(OH)·0.11H2O have not been discovered yet. Herein, we report an important new property of hollow urchin-like Co(CO3)0.5(OH)·0.11H2O for highly selective electrochemical reduction of carbon dioxide to methanol in NaHCO3 aqueous solution at −0.98 V versus saturated calomel electrode (SCE) with Faradaic efficiency of up to 97.0% under ambient conditions, which is superior to most of the electrocatalysts reported to date. Finally, this low-cost electrocatalyst shows great potential in CO2 conversion industry for practical application in the future.
- This article is part of the themed collection: 2018 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...