Green asymmetric synthesis of Warfarin and Coumachlor in pure water catalyzed by quinoline-derived 1,2-diamines†
Abstract
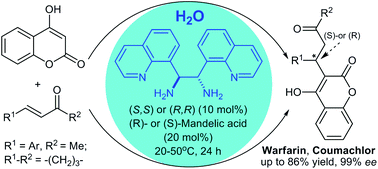
Simple enantiomerically pure C2-symmetric quinoline (isoquinoline)-derived 1,2-diamines were synthesized from the corresponding aldehydes via stereospecific diaza-Cope rearrangement with 2,2′-(1,2-diaminoethane-1,2-diyl)diphenol (HPEN). Efficient green synthesis of both enantiomers of the anticoagulant Warfarin and rodenticide Coumachlor was achieved in an aqueous medium via the asymmetric iminium-type Michael reaction in the presence of the catalysts 8e and (ent)-8e in combination with (R)- or (S)-mandelic acid, respectively. This procedure provides high enantioselectivity (up to 91% ee), which has never been attained for these bioactive compounds with the known catalysts under aqueous conditions. Nearly optically pure Warfarin (∼99% ee) was prepared via a green isolation procedure, which included acidic precipitation of the crude product from a basic aqueous solution followed by single recrystallization. Furthermore, unlike the known primary amine-derived organocatalysts, the developed aqueous catalytic system does not produce parasitic byproducts and can be recovered and reused in the asymmetric reaction.


 Please wait while we load your content...
Please wait while we load your content...