Paired electrochemical conversion of nitroarenes to sulfonamides, diarylsulfones and bis(arylsulfonyl)aminophenols†
Abstract
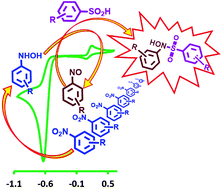
A paired electrochemical method using nitrobenzene (NB) derivatives and arylsulfinic acids (ASAs) as starting materials was developed for the synthesis of some new sulfonamides, diarylsulfones and bis(arylsulfonyl)aminophenols. The synthetic strategy was designed using the data provided by electrochemical studies involving cyclic voltammetry on NB oxidation in the absence and presence of ASAs. The reactions have been successfully performed in an undivided cell, at carbon rod electrodes, in aqueous solutions, by constant current electrolysis at room temperature. This strategy does not require catalysts, toxic solvents and challenging workups. It is also applicable for a wide range of nitroarenes.


 Please wait while we load your content...
Please wait while we load your content...