Abstract
Although N-acyloxazolidinone-based (catalytic) asymmetric synthetic methodologies occupy an important position in modern organic synthesis, the catalytic cleavage of a chiral auxiliary remains underdeveloped. We report the Ni(cod)2/bipyr.-catalyzed alcoholysis of N-acyloxazolidinones to deliver esters. The reaction is broad in scope for both N-acyloxazolidinone substrates and alcohol nucleophiles, and displays good functional group tolerance and excellent chemoselectivity. A gram-scale methanolysis allowed the enantioselective synthesis of the C22–C26 segment of a close analogue of the potent immunosuppressant agent FK506.
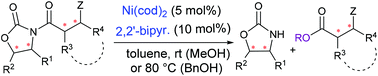
- This article is part of the themed collection: 2018 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...