The synergistic catalysis effect within a dinuclear nickel complex for efficient and selective electrocatalytic reduction of CO2 to CO†
Abstract
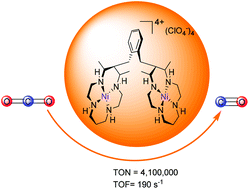
Developing cheap and earth-abundant catalysts for an efficient and selective reduction of CO2 is a promising approach to cut down the increasing emissions of CO2 and obtain valuable fuels/chemicals simultaneously. Here, we present a dinuclear nickel complex, [Ni2L1](ClO4)4 (1, L1 = 1,2-bis((5,7-dimethyl-1,4,8,11-tetraazacyclotetradecan-6-yl)methyl)benzene), which shows an excellent performance for the electrocatalytic reduction of CO2 to CO, with a Faradaic efficiency of 95%, and turnover number (TON) and turnover frequency (TOF) values of 4.1 × 106 and 190.0 s−1, respectively. Electrochemical experiments and density functional theory (DFT) calculations revealed that the excellent catalytic performance of 1 is attributed to the synergistic catalysis effect between two Ni centers within 1.


 Please wait while we load your content...
Please wait while we load your content...