Synthesis of functional catechols as monomers of mussel-inspired biomimetic polymers†
Abstract
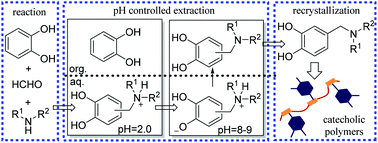
Catechol-containing polymers have attracted much attention in recent years because of their versatile mussel-inspired adhesive function. We report herein the facile synthesis of 4-/3-substituted catechols using the Mannich reaction of catechol with formaldehyde and secondary amines. The reaction proceeds smoothly in water without any catalyst. Separation of the desired products is important for the success of this methodology, and was achieved by a pH-controlled solvent extraction and recrystallization process. The synthesized functional catechols not only exhibited dopamine-like oxidative cross-linking, but also possessed functional groups that are useful for the synthesis of catechol-containing polymers. As a proof-of-concept application, two functional catechols, one bearing monohydroxyl and the other bearing dihydroxyl groups at the 4-aminomethyl substituent, were incorporated into polyacrylate and polyurethane backbones, respectively, endowing these polymers with excellent coatability on various surfaces.


 Please wait while we load your content...
Please wait while we load your content...