Metal-free tandem cyclization/hydrosilylation to construct tetrahydroquinoxalines†
Abstract
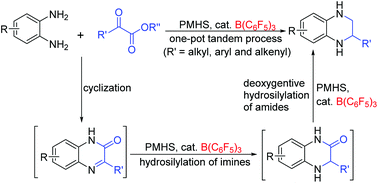
A one-pot tandem procedure involving cyclization and sequential hydrosilylation of imines and amides under the catalysis of B(C6F5)3 has been developed for the step-economical construction of 1,2,3,4-tetrahydroquinoxalines directly from readily available 1,2-diaminobenzenes, α-ketoesters and low-cost, safe polymethylhydrosiloxane (PMHS). This metal-free approach provides various products in good to excellent yields, and displays a wide range of substrate scope and a high degree of functional group tolerance even to reduction-sensitive moieties. The choice of hydrosilanes is critical to the catalysis, and PMHS has proved to be optimal. Decreasing the amount of PMHS could enable the reaction to stop at the 3,4-dihydroquinoxalin-2(1H)-one stage. The procedure is convenient and scalable, and neither a dried solvent nor an inert atmosphere is required. Moreover, the enantioselective construction of these products was explored, and promising results were achieved.
- This article is part of the themed collections: Celebrating our 2020 Prize and Award winners and 2018 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...