Waste-minimised copper-catalysed azide–alkyne cycloaddition in Polarclean as a reusable and safe reaction medium†
Abstract
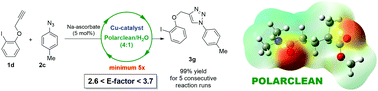
Herein we report the first example of a generally useful organic reaction, namely the copper-catalysed azide–alkyne cycloaddition, performed in a Polarclean/water mixture as a reaction medium. The process is very efficient, affording in 24 out of the 26 tested cases the desired triazole in quantitative yields. Product isolation is also very convenient, since the triazoles either precipitate or form a separate liquid phase, without the need to perform chromatographic separations. Moreover, since the metal catalyst is retained in the Polarclean/water phase, the catalyst/reaction medium can be easily reused for consecutive reaction runs, without an apparent loss in efficiency. This methodology is associated with very limited waste production, as evidenced by calculated E-factors in the range 2.6–3.7.
- This article is part of the themed collection: 2018 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...