An eco-compatible pathway to new hydrotropes from tetraols†
Abstract
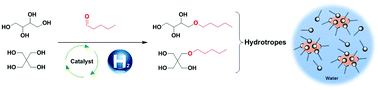
New hydrotropes such as mono-ethers of tetraols were synthesized using an eco-friendly methodology. The mono-ethers, with chain lengths between 5 and 12 carbons, were obtained via a two-step synthesis: acetalisation and hydrogenolysis. Acetalisation, with the corresponding aldehyde, gave rise to 69% to 91% isolated yields of erythritol acetals, and 63% to 79% yields of pentaerythritol acetals. Hydrogenolysis of these acetals was performed resulting in good yields of erythritol (72% to 86%) and pentaerythritol (78% to 83%) derivatives. The physico-chemical analysis of these ethers was realized using DSC and TGA analysis to determine the melting and boiling point. The solubility of the ethers decreased from the alkyl chain of above 6 carbons, with higher hydrophilicity for an erythritol polar head. Salt-tolerance of the polyol ethers showed insensitivity of C5-O- and C6-O-erythritol toward NaCl. C6-O-erythritol and C5-O-pentaerythritol demonstrated better solubilizing properties of Dispers Red 13 compared to conventional hydrotropes. Aqueous self-aggregation was observed at a lower concentration of Cx-O-pentaerythritol compared to Cx-O-erythritol.
- This article is part of the themed collection: 2018 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...