MALDI-TOF-MS characterization of N-linked glycoprotein derived from ginger with ACE inhibitory activity
Abstract
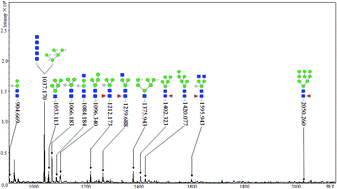
Herein, the ability of ginger glycoproteins to inhibit the angiotensin-converting enzyme (ACE) is characterized. The activity is monitored via HPLC, and then the crude glycoproteins are enriched with lectin microarrays and magnetic microspheres. The N-linked glycans released from the enriched glycoproteins by PNGase F are identified by MALDI-TOF-MS. The results suggest that the crude ginger glycoproteins are active against ACE with an IC50 value of 0.83 ± 0.09 mg mL−1. The ginger glycoproteins are enriched by concanavalin A (Con A) and solanum tuberosum (Potato) lectin (STL), and the structures of the N-glycans released from the ginger glycoproteins include high-mannose type glycans, fucosylated-type glycans, and hybrid-type glycans, as analyzed by MALDI-TOF-MS. The results of this study are expected to provide a reference for the glycan structure of ginger glycoproteins with ACE-inhibitory activity.


 Please wait while we load your content...
Please wait while we load your content...