Chaperone-like food components: from basic concepts to food applications
Abstract
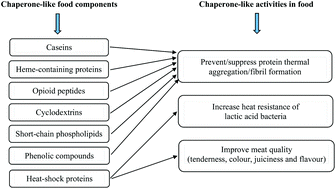
The significance of chaperones in preventing protein aggregation including amyloid fibril formation has been extensively documented in the biological field, but there is limited research on the potential effect of chaperone-like molecules on food protein functionality and food quality. This review is intended to extend the potential of chaperone and chaperone-like molecules in the prevention of food protein aggregation and amyloid fibril formation. The common features of chaperone molecules responsible for suppressing aggregation and amyloid fibril formation are firstly presented. Then, the function and applicability of chaperone and chaperone-like molecules in food products, in particular high-protein beverages where aggregation and fibril formation are undesirable, are extensively discussed. Protein aggregation in high-protein beverages can be prevented by chaperone or chaperone-like components, such as caseins, heme-containing proteins, specific peptide fragments, phospholipids and polyphenols. The diversity of chaperone-like components and mechanisms might provide the flexibility in taking advantage of their potential applications in different food products.


 Please wait while we load your content...
Please wait while we load your content...