Electron-driven proton transfer enables nonradiative photodeactivation in microhydrated 2-aminoimidazole†
Abstract
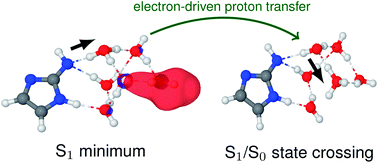
2-Aminoimidazole (2-AIM) was proposed as a plausible nucleotide activating group in a nonenzymatic copying and polymerization of short RNA sequences under prebiotically plausible conditions. One of the key selection factors controlling the lifespan and importance of organic molecules on early Earth was ultraviolet radiation from the young Sun. Therefore, to assess the suitability of 2-AIM for prebiotic chemistry, we performed non-adiabatic molecular dynamics simulations and static explorations of potential energy surfaces of the photoexcited 2-AIM–(H2O)5 model system by means of the algebraic diagrammatic construction method to the second order [ADC(2)]. Our quantum mechanical simulations demonstrate that 1πσ* excited states play a crucial role in the radiationless deactivation of the UV-excited 2-AIM–(H2O)5 system. More precisely, electron-driven proton transfer (EDPT) along water wires is the only photorelaxation pathway leading to the formation of 1πσ*/S0 conical intersections. The availability of this mechanism and the lack of destructive photochemistry indicate that microhydrated 2-AIM is characterized by substantial photostability and resistance to prolonged UV irradiation.
- This article is part of the themed collection: Quantum effects in small molecular systems


 Please wait while we load your content...
Please wait while we load your content...