Towards the systematic crystallisation of molecular ionic cocrystals: insights from computed crystal form landscapes†
Abstract
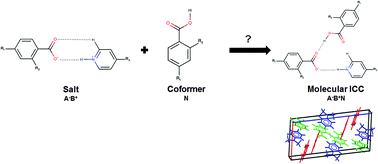
The underlying molecular and crystal properties affecting the crystallisation of ionic cocrystals (ICCs) with the general formula A−B+N (A− = anion, B+ = cation and N = neutral acid molecule; 1 : 1 : 1 stoichiometry) are reported for a limited set of known crystal structures determined following the cocrystallisation of either 4-aminopyridine (which forms salts) or 4-dimethylaminopyridine (which forms salts and ICCs) with the same set of monoprotic acids with a single hydroxy or halogen substitution at the ortho or para position. Periodic density functional theory calculations (PBE + D2) on the energetic driving force for ICC crystallisation for a set of known crystal structures with well characterised acid, salt and ICC structures show that all but 1 of the 7 experimental ICC structures surveyed were more stable than the sum of their component salt and acid structures with 4 displaying relative stabilities (ΔEICC) ranging from 2.47–8.02 kJ mol−1. The majority of molecular ICCs that are more stable with respect to their component salt and acid structures display the formation of discrete intermolecular O–Hacid⋯Oanion hydrogen bonds with the D11(2) graph set between the carboxylic acid OH donor and the carboxylate oxygen acceptor of the anion. Computed crystal form landscapes for model 1 : 1 salts derived from acid–base pairs (involving 4-dimethylaminopyridine) known to form molecular ICCs show that on average the most stable predicted polymorphs of the 1 : 1 salts have efficient packing of the ions with packing coefficients in the range 65–80% and this is comparable to the packing coefficients of the most stable predicted polymorphs of 1 : 1 salts (involving 4-aminopyridine) that have no ICCs reported. This suggests that the cocrystallisation of equimolar amounts of the 1 : 1 salt and the acid to form a 1 : 1 : 1 molecular ICC is a complicated phenomenon that cannot be explained on the basis of inefficiencies in the crystal packing of the salt ions.
- This article is part of the themed collection: Methods and applications of crystal structure prediction


 Please wait while we load your content...
Please wait while we load your content...