Computational studies on ground and excited state charge transfer properties of peptidomimetics†
Abstract
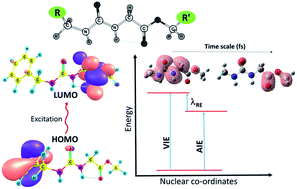
Chemical modifications at various peptide positions result in peptidomimetics with unique physical and chemical properties that can be used for a range of applications. Among many peptidomimetics, ureidopeptides are interesting due to their ability to act as donor–bridge–acceptor systems through which charge transfer occurs in one direction and can be triggered by an electrochemical pulse without perturbing the nuclear position. In this regard, some UP mimetics with different chromophoric units are studied in this work to understand their role using DFT based methods. Computational results and natural charge analysis provide evidence for the extensive contribution of the substituents to the excitation and hole migration dynamics. Further, the results show that the UP backbone preserves its uni-directional charge transfer phenomenon from the ureido to carboxylate terminal irrespective of the terminal groups and position. However, the substituent affects the excitation energies and the time scales of the hole migration. Among the substituents studied here, fluorine migrates to the hole within a shorter time scale while phenyl groups take longer.
- This article is part of the themed collection: Photoinduced Processes in Nucleic Acids and Proteins


 Please wait while we load your content...
Please wait while we load your content...