Applying neutron diffraction with isotopic substitution to the structure and proton-transport pathways in protic imidazolium bis{(trifluoromethyl)sulfonyl}imide ionic liquids
Abstract
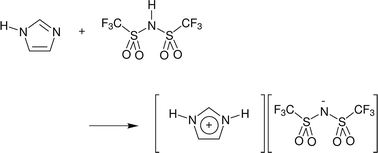
Neutron diffraction with isotopic substitution has been applied to examine the potential for complex-ion formation in protic imidazolium bis{(trifluoromethyl)sulfonyl}imide ionic liquids. Strong cation–anion hydrogen-bonding in the 1 : 1 base : acid ionic liquid results in a high population of anions adopting a cis-conformation and, on adding excess imidazole (2 : 1 base : acid stoichiometry), cation–base and base–base correlations were identified, however, persistent hydrogen-bond associations were not observed.
- This article is part of the themed collection: Ionic liquids: from fundamental properties to practical applications


 Please wait while we load your content...
Please wait while we load your content...