Emerging investigators series: comparing the inherent reactivity of often-overlooked aqueous chlorinating and brominating agents toward salicylic acid†
Abstract
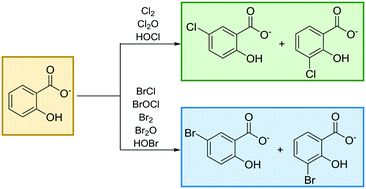
Chlorinated and brominated forms of salicylic acid (SA) have recently been identified as a new class of disinfection byproducts (DBPs) in drinking water. Herein, we report the inherent reactivity of several aqueous halogenating agents toward hydrogen salicylate, the predominant species of salicylic acid under environmental conditions. Using synthetic waters, halogenation rates associated with the formation of 3-chloro, 5-chloro, 3-bromo, and 5-bromosalicylate were measured as a function of pH, [Cl−], [Br−], free chlorine dose, and the initial concentration of SA. Halogenating-agent specific second-order rate constants were determined and decrease in the order: BrCl > BrOCl > Br2 > Br2O > Cl2 > Cl2O > HOBr > HOCl. Chloride is capable of enhancing rates of bromination and chlorination, ostensibly by promoting the formation of BrCl and Cl2, both of which are several orders of magnitude more inherently reactive than HOBr and HOCl, respectively. Kinetic data also support the participation of salicyloyl hypochlorite as a chlorination intermediate capable of influencing chlorination rates at pH >8. Experiments in which buffer concentrations were varied indicate that phosphate buffers can enhance rates of SA bromination but not chlorination; carbonate and borate buffers did not appreciably influence rates of bromination or chlorination. Under conditions representative of chlorinated drinking water, rates of SA bromination will generally exceed rates of SA chlorination. The results discussed herein demonstrate the importance of considering halogenating agents beyond HOBr and HOCl when developing kinetic models to describe and predict halogenation rates and selectivity in waters containing free chlorine.
- This article is part of the themed collections: Emerging Investigator Series and Best Papers 2018 – Environmental Science: Water Research & Technology


 Please wait while we load your content...
Please wait while we load your content...