Ligand-dependent Ag2S formation: changes in deposition of silver nanoparticles with sulfidation†
Abstract
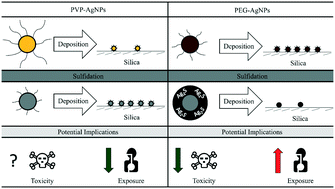
The surfaces of nanoparticles (NPs) are continuously evolving as they are exposed to different environmental conditions. Under the same sulfidation conditions, silver NPs (AgNPs) are generally considered to undergo the same surface modifications (i.e., removal of original ligands and formation of a new silver sulfide (Ag2S) shell). We examined this generalization by studying how different ligands, polyvinylpyrrolidone (PVP) and thiolated polyethylene glycol (PEG), affected 1) the formation of a new Ag2S shell and 2) the subsequent mobility of these transformed AgNPs. The deposition of PEG-AgNPs onto a silica substrate decreased by a factor of 40 after sulfidation, while the deposition of PVP-AgNPs increased by a factor of 15. The decreased deposition of sulfidized PEG-AgNPs was attributed to the removal of PEG and the formation of Ag2S, which created a more negatively charged surface (−15 mV to −30 mV) and consequently greater electrostatic repulsion with the silica substrate. The increased deposition of sulfidized PVP-AgNPs was suggested to be caused by the removal of PVP and the absence of Ag2S, which decreased steric repulsion with the silica substrate. For the first time, this study revealed the unique abilities of two common polymeric coatings of AgNPs to form Ag2S during sulfidation and how Ag2S determined their subsequent mobility. The results of this communication also highlighted the importance of monitoring the continuous evolution of the NP surface for better prediction of NP exposure and toxicity.


 Please wait while we load your content...
Please wait while we load your content...
