Reaction of bisphenol A with synthetic and commercial MnOx(s): spectroscopic and kinetic study†
Abstract
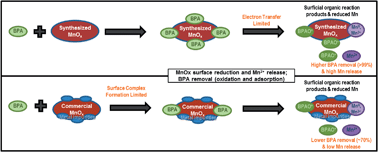
The reaction of bisphenol A (BPA) using laboratory synthesized (Syn-MnOx) and commercially available (Com-MnOx) MnOx(s) media was investigated using spectroscopic and aqueous chemistry methods. The surface area of Syn-MnOx (128 m2 g−1) and Com-MnOx (13.6 m2 g−1) differed by an order of magnitude. The impurities were less than 1% by weight for Syn-MnOx while Com-MnOx contained 29% impurity by weight, mainly Al, Si and Fe. The removal of 99.7% BPA was observed applying Syn-MnOx, while 71.2% BPA removal was observed applying Com-MnOx after 44 hours of reaction of 10 mM MnOx(s) media with 1 mM BPA at pH 5.5. The reduction of Mn was detected in the surface of both BPA reacted media, but a higher content of reduced Mn was observed in Syn-MnOx (52% in Syn-MnOx compared to 29% in Com-MnOx). The release of soluble Mn was an order of magnitude higher in batch experiments reacting BPA with Syn-MnOx compared with Com-MnOx. The C 1s and O 1s XPS high resolution spectral analyses identified the presence of functional groups that likely correspond to BPA oxidation products, such as dimers and quinones associated with MnOx(s) surfaces on both reacted media. The reaction of BPA with Syn-MnOx fit the electron transfer-limited model (R2 = 0.96), while the reaction of BPA with Com-MnOx had a better fit for surface complex formation-limited model (R2 = 0.95). These results suggest that BPA removal and the reactivity of MnOx(s) are affected by the differences in surface area and impurities present in these media. Thus, this study has relevant implications for the reaction of MnOx(s) with emerging organic contaminants in natural biogeochemical processes and water treatment applications.


 Please wait while we load your content...
Please wait while we load your content...
