Investigation on the hygroscopicity of oxalic acid and atmospherically relevant oxalate salts under sub- and supersaturated conditions
Abstract
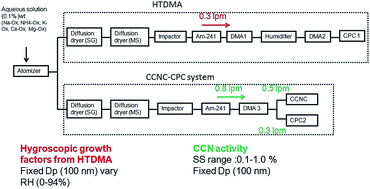
Oxalic acid (OxA) is an end product in the oxidation of many organic compounds, and therefore is ubiquitous in the atmosphere and is often the most abundant organic species in ambient aerosols. To better understand the hygroscopic properties of OxA under sub- and supersaturated conditions in the atmosphere, we investigated the hygroscopic growth and cloud condensation nuclei (CCN) activation ability of pure OxA and its salts using a hygroscopic tandem differential mobility analyzer (HTDMA) and cloud condensation nuclei counter (CCNC), respectively. OxA particles absorb water under >45% RH, suggesting that the initial phase state might be an amorphous solid. The measured hygroscopic growth factor (HGF) of OxA at 90% RH was 1.47. We found that the HGF of ammonium oxalate (NH4-Ox) was larger than that of OxA, whereas HGFs of sodium, calcium, and magnesium oxalates (Na-Ox, Ca-Ox, and Mg-Ox) were smaller than that of OxA particles. Potassium oxalate (K-Ox) behaved like a typical water-soluble inorganic salt, exhibiting deliquescence and efflorescence transitions at around 85% and 50% RH, respectively. Na-Ox exhibited strong activation capabilities among all the investigated salts, followed by NH4-Ox and K-Ox as inferred from the activation ratios (CCN/CN) against supersaturations (SS). On the other hand, Ca-Ox showed moderate activation ability and Mg-Ox showed poor CCN activation ability. We also observed significantly higher κCCN values compared to κHTDMA for pure OxA and its salts (NH4-Ox and Na-Ox), suggesting that the condensation of OxA into the aqueous phase occurs during water uptake. These findings improve the fundamental understanding of hygroscopic behaviors and phase states of oxalic acid and its salts under sub- and supersaturated conditions in the atmosphere and impacts of hygroscopicity on the direct and indirect effects of aerosol particles.


 Please wait while we load your content...
Please wait while we load your content...