Intermediate temperature fuel cells via an ion-pair coordinated polymer electrolyte†
Abstract
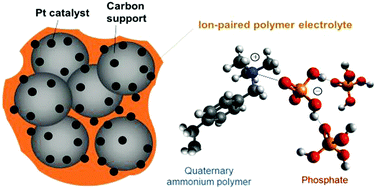
Fuel cells are attractive devices that convert chemical energy into electricity through the direct electrochemical reaction of hydrogen and oxygen. Intermediate temperature fuel cells operated at 200–300 °C can simplify water and thermal managements, enable the use of non-precious or low-loading precious metal catalysts and provide insensitivity toward fuel and air impurities such as carbon monoxide. However, the performance of current intermediate temperature fuel cells is poor due to a lack of highly-conductive membrane electrolytes and optimal electrodes designed for these fuel cells. Here, we demonstrate high-performing intermediate temperature fuel cells that use SnP2O7–polymer composite membranes and a quaternary ammonium-biphosphate ion-pair coordinated polymer electrolyte in the electrodes. The peak power density of the fuel cell under H2 and O2 reached 870 mW cm−2 at 240 °C with minimal performance loss under exposure to 25% carbon monoxide.


 Please wait while we load your content...
Please wait while we load your content...
