Calcium cobaltate: a phase-change catalyst for stable hydrogen production from bio-glycerol†
Abstract
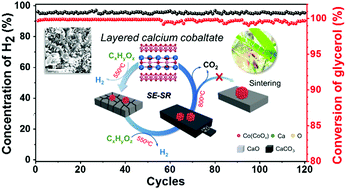
The sorption enhanced steam reforming (SESR) technology has the potential to produce high purity hydrogen by Le Chatelier's principle. However, its practical applicability is limited by sorbent sintering and deactivation at high reaction/decarbonation temperatures. Herein, we propose a novel strategy to enhance the stability of the SESR of glycerol (SESRG), in which misfit layered materials, i.e. calcium cobaltates (CCO), were used as a dual-functional material combining CO2 absorption and catalytic reforming. Differing from the conventional approach of enhancing the robustness of catalysts/sorbents, we exploited the reversible phase change of CCO: Ca3Co4O9 ↔ Co + CaO, during the decarbonation and reaction steps respectively. By doing so, the sintering of the CaO sorbent and the Co catalyst could be suppressed because they were homogenized into CCO on an atomic level in every decarbonation stage. The CCO catalyst displayed a very stable performance for producing high purity H2 through SESRG for up to 120 reaction–decarbonation cycles, without noticeable changes in H2 production and CO2 absorption capacity. In situ XRD and microscopy studies demonstrated the reversible phase transition and the accompanied formation of hierarchical CCO micro-structures that facilitated the catalytic reforming and CO2 absorption, benefited from the complex phase equilibria among different CCO compounds. The results in this study shed light on a new paradigm for the design of materials working at high temperatures thus suffering from serious sintering.


 Please wait while we load your content...
Please wait while we load your content...