Lithium manganese oxyfluoride as a new cathode material exhibiting oxygen redox†
Abstract
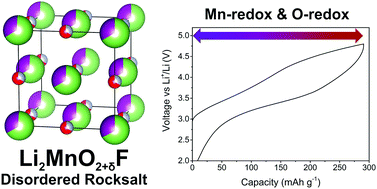
The quantity of charge stored in transition metal oxide intercalation cathodes for Li or Na batteries is not limited by transition metal redox reactions but can also access redox reactions on O; examples include Li1.2Ni0.13Mn0.54Co0.13O2, Li2Ru0.75Sn0.25O3, Li1.2Nb0.3Mn0.4O2, Na2RuO3 and Na2/3Mg0.28Mn0.72O2. Here we show that oxyfluorides can also exhibit charge storage by O-redox. We report the discovery of lithium manganese oxyfluoride, specifically the composition, Li1.9Mn0.95O2.05F0.95, with a high capacity to store charge of 280 mA h g−1 (corresponding to 960 W h kg−1) of which almost half, 130 mA h g−1, arises from O-redox. This material has a disordered cubic rocksalt structure and the voltage–composition curve is significantly more reversible compared with ordered Li-rich layered cathodes. Unlike lithium manganese oxides such as the ordered layered rocksalt Li2MnO3, Li1.9Mn0.95O2.05F0.95 does not exhibit O loss from the lattice. The material is synthesised using a simple, one-pot mechanochemical procedure.


 Please wait while we load your content...
Please wait while we load your content...