Phenylacetylene polymerisation mediated by cationic cyclometallated palladium(ii) complexes bearing benzylidene 2,6-diisopropylphenylamine and its derivatives as ligands†
Abstract
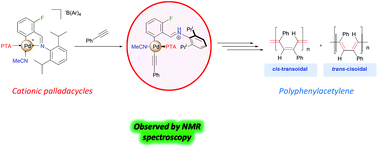
A series of novel cationic palladacycle complexes bearing benzylidene-2,6-diisopropylphenylamine derivatives as ligands and with the general formula [Pd(MeCN)(L)(R-C6H3)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N{2,6-iPr2-C6H3}][B(3,5-(CF3)2-C6H3)] (R = H, Cl, Br, F, OMe and L = 1,3,5-triaza-7-phosphaadamantane (PTA), tricyclohexylphosphine (PCy3) and triphenylphosphine (PPh3)) were prepared and characterized by a range of analytical techniques. These cationic palladacycle complexes were found to be active precatalysts for the polymerisation of phenylacetylene. The meta-substituent on the cyclometallated ring was found to have a marked effect on the catalyst performance in that complexes, which contained electron-withdrawing substituents, were found to be the most active in the polymerisation process. Furthermore, increasing the steric bulk of the phosphine ligand led to the production of higher molecular weight polyphenylacetylenes (PPA). Polymerisation reactions performed at 25 °C gave a mixture of both cis-transoidal and trans-cisoidal PPA while trans-cisoidal PPA was selectively produced at elevated temperatures (60 °C). Preliminary mechanistic studies demonstrated that polymerisation proceeds via dissociation of the C,N-chelating ligand, and involves the formation of an iminium cationic fragment.
N{2,6-iPr2-C6H3}][B(3,5-(CF3)2-C6H3)] (R = H, Cl, Br, F, OMe and L = 1,3,5-triaza-7-phosphaadamantane (PTA), tricyclohexylphosphine (PCy3) and triphenylphosphine (PPh3)) were prepared and characterized by a range of analytical techniques. These cationic palladacycle complexes were found to be active precatalysts for the polymerisation of phenylacetylene. The meta-substituent on the cyclometallated ring was found to have a marked effect on the catalyst performance in that complexes, which contained electron-withdrawing substituents, were found to be the most active in the polymerisation process. Furthermore, increasing the steric bulk of the phosphine ligand led to the production of higher molecular weight polyphenylacetylenes (PPA). Polymerisation reactions performed at 25 °C gave a mixture of both cis-transoidal and trans-cisoidal PPA while trans-cisoidal PPA was selectively produced at elevated temperatures (60 °C). Preliminary mechanistic studies demonstrated that polymerisation proceeds via dissociation of the C,N-chelating ligand, and involves the formation of an iminium cationic fragment.


 Please wait while we load your content...
Please wait while we load your content...