Modular bimetallic complexes with a sulfonamido-based ligand†
Abstract
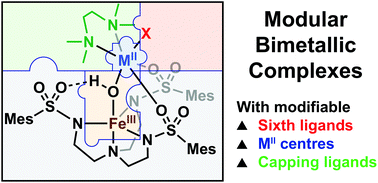
A series of bimetallic complexes prepared with the ligands N,N,N′,N′-tetramethylethane-1,2-diamine (TMEDA) and N,N′,N′′-[2,2′,2′′-nitrilotris(ethane-2,1-diyl)]tris(2,4,6-trimethylbenzenesulfonamido) ([MST]3−) is described. Four diiron compounds of the formulation (TMEDA)FeII(X)-(μ-OH)-FeIIIMST were prepared, in which the X− ligands are the anions OTf−, Br−, SCN−, or N3−. Additionally, two heterobimetallic compounds of the formulation (TMEDA)MII(OTf)-(μ-OH)-FeIIIMST (MII = CoII or NiII) were synthesized. All these compounds have similar spectroscopic and structural properties. The diiron compounds exhibit perpendicular-mode electron paramagnetic resonance spectra consistent with S = 1/2 spin ground states, which is expected for high-spin FeII and FeIII centres that are antiferromagnetically coupled. The heterobimetallic (TMEDA)NiII(OTf)-(μ-OH)-FeIIIMST complex had a spin state of S = 3/2 that also resulted from antiferromagnetic coupling between the high-spin NiII and FeIII centres. The modularity of this system is further demonstrated by the substitution of the TMEDA ligand with ethylenediamine (en); for this species two equivalents of en coordinate to the FeII centre to form [(en)2FeII-(μ-OH)-FeIIIMST]OTf. These results demonstrate that a modular bimetallic system has been developed in which the key components can be modified.


 Please wait while we load your content...
Please wait while we load your content...