Synthesis and characterisation of fluorescent aminophosphines and their coordination to gold(i)†
Abstract
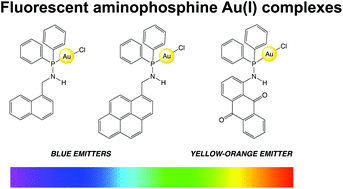
Three novel fluorescent aminophosphine ligands have been synthesised that incorporate napthyl (L1), pyrenyl (L2) and anthraquinone (L3) chromophores into their structures. The ligands react with [AuCl(tht)] (tht = tetrahydrothiophene) to give neutral complexes of the form [AuCl(L1–3)]. Solid state, X-ray crystallographic data was obtained for the anthraquinone derivative, [AuCl(L3)], and showed a distorted linear coordination geometry at Au(I). The packing structure also revealed a number of intermolecular π–π interactions that involve the anthraquinone and phenyl units of the aminophosphine ligand. 31P NMR spectroscopic data revealed δP values of +42.2 (L1), +42.1 (L2) and +26.1 (L3) ppm, which shifted downfield upon coordination to Au(I) to +64.6, +64.7, and +55.8 ppm, respectively. Supporting TD-DFT studies were able to reproduce the structure and 31P NMR chemical shifts of [AuCl(L3)] as well as rationalise the HOMO–LUMO compositions. Photophysical studies showed that the appended fluorophore dominates the absorption and emission properties for the ligands and complexes, with the anthraquinone derivatives showing visible emission at ca. 570 nm which was attributed to the intramolecular charge transfer character of the phosphinoaminoanthraquinone fragment.


 Please wait while we load your content...
Please wait while we load your content...