Novel enantiopure monophospholes: synthesis, spatial and electronic structure, photophysical characteristics and conjugation effects†
Abstract
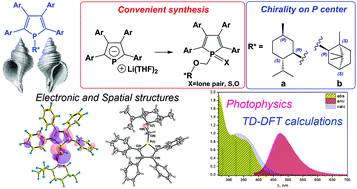
A rational and highly efficient method to access lithium 2,3,4,5-tetraphenylphospholide directly from white phosphorus, diphenylacetylene and lithium has been developed. This novel, convenient synthetic route has allowed the incorporation of various chiral substituents into 2,3,4,5-tetraarylphospholes. The spatial and electronic structures of the obtained novel enantiopure phospholes, their oxides and sulfides have been characterized both experimentally (single-crystal X-ray diffraction) and computationally (DFT). The experimental vibrational Raman spectra and electronic absorption and emission spectra of the synthesized compounds have been interpreted using quantum chemical calculations. A moderate impact of the substituent at the P-atom on the photophysical properties of the phospholes was demonstrated both experimentally and theoretically, while the oxidation/thionation of the phosphorus center was shown to influence remarkably both absorption and emission spectral characteristics. The latter effect is ascribed to the increase of conjugation strength of the oxidized/thionated species with exocyclic Ph rings.


 Please wait while we load your content...
Please wait while we load your content...