Coordination polymers from alkaline-earth nodes and pyrazine carboxylate linkers†
Abstract
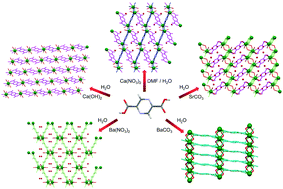
A new series of alkaline-earth-metal based coordination polymers were synthesized by using a pyrazine-2,5-dicarboxylic acid (2,5-H2pzdc) ligand under hydrothermal conditions. These compounds show a variety of structural topologies, reflecting the variable coordination geometries of the alkaline-earth ions as well as the key role of the metal precursor salts. Ca, Sr, and Ba give porous three-dimensional compounds, namely [Ca(2,5-pzdc)(H2O)2]·H2O (1), [Sr(2,5-pzdc)(H2O)4]·H2O (3), [Ba(2,5-pzdc)(H2O)4]·2H2O (4) and [Ba(2,5pzdc)(H2O)2] (5), that feature one-dimensional hydrophilic channels which are filled with water molecules. The Sr compound retains its structure when the lattice water molecules are removed, while the other compounds undergo a structural rearrangement. The hydrophilicity of the Sr compound combined with its high stability even in the absence of guest molecules are the key characteristics that determine its good water adsorption and proton conductivity properties.


 Please wait while we load your content...
Please wait while we load your content...