Co-synthesis of atomically precise nickel nanoclusters and the pseudo-optical gap of Ni4(SR)8†
Abstract
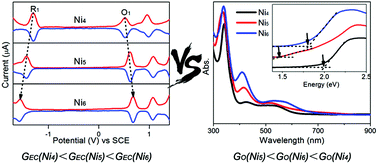
Three sizes of atomically precise tiara-like structural Nin(SR)2n (n = 4, 5 and 6) are co-synthesized by a one-pot method and isolated using thin layer chromatography. The molecular formulas of Ni5(SR)10 and Ni6(SR)12 are determined using matrix-assisted laser desorption ionization mass spectrometry, and the tiara-like structures of Ni4(SR)8 and Ni6(SR)12 are proved using single-crystal X-ray crystallography. Nuclear magnetic resonance shows that Ni5(SR)10 has a similar tiara-like structure too. The electrochemical gap enlarges with the increase of the cluster size, which agrees with the calculated HOMO–LUMO gap and optical gap by TD-DFT, but the experimental optical gap is GO(Ni4(SR)8) > GO(Ni6(SR)12) > GO(Ni5(SR)10) due to Ni4(SR)8 owning a pseudo-optical gap at 2.0 eV.


 Please wait while we load your content...
Please wait while we load your content...