Water oxidation by a copper(ii) complex: new findings, questions, challenges and a new hypothesis†
Abstract
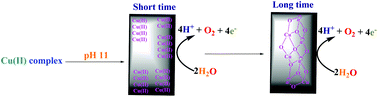
Copper(II) complexes are very promising catalysts for water oxidation. Herein new findings on the water-oxidizing activity of a few copper(II) complexes under water oxidation conditions are reported. Copper compounds in this study are copper(II) phthalocyanine-3,4′,4′′,4′′′-tetrasulfonic acid tetrasodium salt (1), the product from the hydrolysis of Cu(II)tptz(H2O)(CH3COO)2 (tptz: 2,4,6-tris(2-pyridyl)-s-triazine) (2), Cu(II)(phen)(CH3CN)2(ClO4)2 (3), Cu(II)(phen)2(CH3CN)(ClO4)2 (4), and copper(II) sulfate pentahydrate (Cu(II) salt), which were investigated in the context of the water oxidation reaction by electrochemical and related methods. The experiments showed that among these compounds at pH = 11, only Cu(II) salt and 3 led to immediate water oxidation. On the other hand, for stable complexes 1, 2 and 4 even after a few minutes low water oxidation rates were observed. The role of nanosized particles of Cu oxide or Cu ions in electrochemical water oxidation was investigated. Under the water oxidation conditions, the electrode, Cu(II) complexes and Cu(II) salt were studied and a relationship between the stability of the Cu(II) complex and water oxidation rate was suggested. It is proposed that Cu(II) ions or clusters, rather than the starting copper(II) complex or copper(II) oxide, are the true catalysts for the investigated water oxidation process in short-term amperometry. For 3 and in long-term amperometry, CuOx was detected. The experiments showed that a molecular mechanism for the water oxidation reaction in the presence of copper(II) complexes should be carefully analyzed to verify the role of copper ions or cluster formation in the water oxidation reaction.


 Please wait while we load your content...
Please wait while we load your content...