Ferrate(ii) complexes with redox-active formazanate ligands†
Abstract
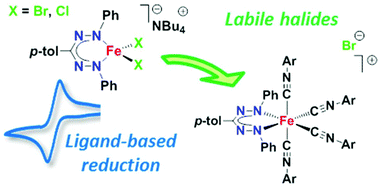
The synthesis of mono(formazanate) iron complexes is described. In the presence of tetrabutylammonium halides, salt metathesis reactions afford the ferrate(II) complexes [Bu4N][LFeX2] (L = PhNNC(p-tol)NNPh; X = Cl, Br) in good yield, and the products are characterized in detail. The high-spin ferrate(II) complexes show cyclic voltammograms that are consistent with reversible, ligand-based one-electron reduction. The halides in these ferrate(II) compounds are labile, and are displaced by 4-methoxyphenyl isocyanide (4 equiv.) as evidenced by formation of the low-spin, cationic octahedral complex [LFe(CNC6H4(p-OMe))4][Br]. Thus, a straightforward route to mono(formazanate) iron(II) complexes is established.


 Please wait while we load your content...
Please wait while we load your content...