Versatile coordination of acetazolamide to ruthenium(ii) p-cymene complexes and preliminary cytotoxicity studies†
Abstract
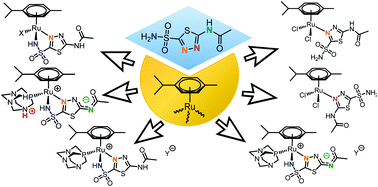
The carbonic anhydrase inhibitor acetazolamide (AcmH2) reacted with [(η6-p-cymene)RuCl(μ-Cl)]2 to afford [(η6-p-cymene)RuCl2(κN-AcmH2)], 1A, in near-quantitative yield. In methanol, 1A exists in equilibrium with 1B, being probably a coordination isomer, as established by VT 1H-EXSY NMR spectroscopy. DFT calculations pointed to a higher stability of 1A with respect to 1B. [(η6-p-cymene)RuCl(κ2N,N′-AcmH)], 2, was obtained in 86% yield from [(η6-p-cymene)RuCl(μ-Cl)]2 and AcmH2 in the presence of NaOH. The reactions of 2 with AgNO3 (in water), pta/AgNO3 or pta/AgOTf/Et3N (in methanol) afforded the nitrate-coordinated complex [(η6-p-cymene)Ru(κO-NO3)(κ2N,N′-AcmH)], 3, the salt [(η6-p-cymene)Ru(κ2N,N′-AcmH)(κP-pta)]NO3, [4]NO3, and the zwitterion [(η6-p-cymene)Ru(κ2N,N′-Acm)(κP-pta)], 5, respectively, in high yields (pta = 1,3,5-triaza-7-phosphatricyclo[3.3.1.1]decane). The reactions of 5 with Brønsted acids yielded the protonated-pta species [(η6-p-cymene)Ru(κ2N,N′-Acm)(κP-ptaH)]X [6]X (X = NO3, TsO). All compounds were fully characterized by analytical and spectroscopic methods, and the structures of 1A, 2 and 5 were elucidated by X-ray diffraction. The stability of the compounds was investigated in aqueous media and 2 and 5 were evaluated for their cytotoxicity towards human ovarian A2780 and A2780cisR cancer cells and non-tumorigenic HEK-293 cells.


 Please wait while we load your content...
Please wait while we load your content...