Switching on the proton transport pathway of a lanthanide metal–organic framework by one-pot loading of tetraethylene glycol for high proton conduction†
Abstract
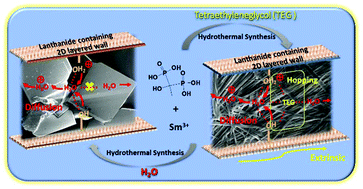
A one-pot hydrothermal approach has been developed to introduce tetraethylene glycol (TEG) molecules into a two-dimensional (2D) layered lanthanide metal–organic framework ([Sm(H5C2P2O7)(H2O)2]·Guest, denoted SmHEDP-Guest). Through the straightforward loading of TEG, the proton conductivity of SmHEDP-TEG (1.21 × 10−3 S cm−1) is increased by 3 orders of magnitude compared with its analogue SmHEDP-H2O (1.22 × 10−6 S cm−1) under 100% relative humidity at room temperature. More excitingly, SmHEDP-TEG exhibits very high proton conductivity of 9.17 × 10−2 S cm−1, even higher than commercial Nafion, when the temperature is increased to 333 K, which is significantly higher than SmHEDP-H2O (3.38 × 10−5 S cm−1). The single crystal XRD reveals that the adjacent water molecules located in the channels of SmHEDP-H2O are isolated without hydrogen bonding interactions owing to their long distances. However, interestingly, the guest TEG molecules of SmHEDP-TEG behave as hydrogen bonded connected bridges, which switch on the proton transport pathway to promote proton hopping. This discovery may provide a facile strategy to design and synthesize more promising candidates for novel proton conductors.


 Please wait while we load your content...
Please wait while we load your content...