Electrochemical water oxidation using a copper complex†
Abstract
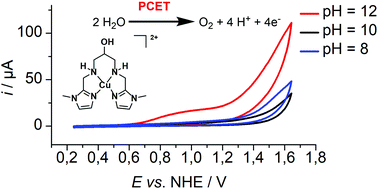
Herein, we report the application of the mononuclear copper complex 1, [CuII(L)]2+, in electrochemical water oxidation catalysis (L = 1,3-bis(((1-methyl-1H-imidazol-2-yl)methyl)amino)propan-2-ol). The complex exhibits a N4 donor set consisting of two amine and two imidazole units and a dangling OH unit in close proximity to the copper ion. 1 exhibits a moderate apparent rate constant kcat of 0.12 s−1 in catalysis and operates at an overpotential of 0.83 V. Detailed investigations allowed us to derive a mechanism for water oxidation. The catalysis proceeds only under basic conditions, where [CuII(L)(OH)]+, 1H−1, is the main solution species, which indicates that a negatively charged ligand is necessary to drive the catalysis. Initial oxidation of 1H−1 is coupled to proton loss forming a copper(III) species and further oxidation initiates oxygen evolution. Initial oxidation of 1 under neutral, i.e. non-catalytic, conditions is pH independent, highlighting the importance of PCET steps during catalysis. We collected reasonable evidence that catalysis proceeds via a water nucleophilic attack mechanism. The electrolyte presumably acts as a proton acceptor in catalysis as the onset potential depends on the buffer employed.
- This article is part of the themed collection: New Talent: Europe


 Please wait while we load your content...
Please wait while we load your content...