A PEG/copper(i) halide cluster as an eco-friendly catalytic system for C–N bond formation†
Abstract
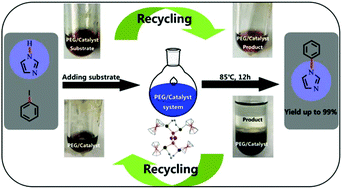
The catalytic activities of eight copper(I) halide clusters assembled from copper(I) halide and ferrocenyltelluroethers, 1–8, were investigated in C–N formation under various conditions. A catalytic procedure using poly(ethylene glycol) (PEG-400) as a greener alternative organic solvent has been developed. The PEG-400/5 system can achieve 99% targeted yield with a mild reaction temperature and short reaction time. After the isolation of the products by extraction with diethyl ether, this PEG-400/cluster system could be easily recycled. Spectroscopic studies elucidate a stepwise mechanism: firstly, proton-coupled electron transfer (PCET) involving the transfer of an electron from Cu+ and a proton from imidazole results in the formation of a labile penta-coordinated Cu2+ and aryl radical; the following effective electron transfer from the ferrocene unit reduces Cu2+ and forms the target product; finally, the ferrocenium unit is reduced by the I− anion. The merits of this eco-friendly synthesis are the efficient utilization of reagents and easy recyclability.


 Please wait while we load your content...
Please wait while we load your content...